Abstract
Background Peripheral T-cell lymphoma (PTCL) is a heterogeneous group of T-cell lymphoma generally characterized by aggressive behaviors. Nodal PTCL encompasses four major subtypes, PTCL-NOS, angioimmunoblastic T cell lymphoma (AITL), and anaplastic large cell lymphoma (ALCL), ALK-positive (pos) or ALK-negative (neg). The role of autologous hematopoietic cell transplant (auto HCT) during the first response post upfront chemotherapy in nodal-PTCL is still debated. The largest prospective cohort trial (COMPLETE) showed that overall survival (OS) was not statistically different between auto HCT during the first remission compared to nontransplant at the median follow-up of 2.8 years (Park et al. Cancer 2019). This study aims to compare the survival outcomes of patients who received auto HCT to those who did not receive auto HCT during the first response in nodal PTCL.
Methods We retrospectively reviewed clinical data on 133 patients at Moffitt Cancer Center who achieved complete response (CR) or partial response (PR) after receiving upfront chemotherapy from 2/2005 to 3/2021. Patients who had stable or progressive disease after the first course of chemotherapy were excluded. Clinical data were abstracted in accordance with institutional review board-approved protocol. Patients were divided into two subgroups: cohort A) patients who received auto HCT during the first response and cohort B) patients who did not receive auto HCT during the first response. The baseline clinical data of two cohorts were summarized using descriptive statistics and compared using Kruskal-Wallis tests for continuous variables and Chi-squared tests for categorical variables. Overall survival (OS) was calculated from the start date of upfront chemotherapy to death or censored to the last follow-up. Median OS (mOS) was calculated using the Kaplan Meier method and compared two cohorts with a log-rank test. Univariate and multivariate COX proportional hazard (PH) analysis was used to evaluate the association of OS.
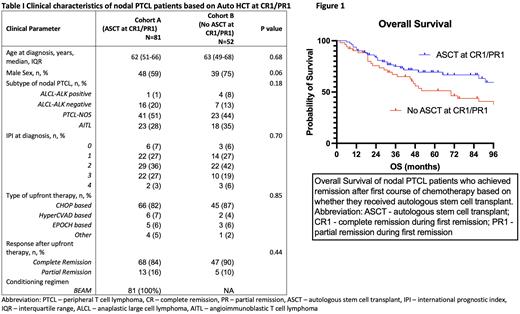
Results Out of 133 patients, 81 (61%) patients were assigned to cohort A. The remaining 52 (39%) patients in cohort B included 30 patients who received auto HCT at CR2 or higher and 22 patients who did not undergo HCT. Of the whole cohort, more than half of the patients (62%) had intermediate-risk diseases based on IPI. The histology subtype included 4% ALK-pos ALCL, 17% ALK-neg ALCL, 48% PTCL-NOS, and 21% AITL. Most patients (83%) in our cohort received CHOP-based therapy upfront and 86% of patients achieved CR after upfront chemotherapy. Clinical characteristics were not significantly different between the two cohorts (table 1).
The mOS for patients in cohort A (auto HCT in CR1/PR1) was significantly higher than cohort B (mOS: not reached [95% CI: 60-131 months] for cohort A, and 70 months [95% CI: 39-101 months] for cohort B, p=0.038). The 3-year OS rate in cohort A was 73% as compared to 43% in cohort B (p=0.18). In the univariate analysis, receiving auto HCT during CR1/PR1 was associated with a hazard ratio (HR) of 0.580 (p=0.042). When adjusted for age at diagnosis, sex, histology subtype, types of induction therapy, and response after upfront chemotherapy in the multivariate analysis, receiving auto HCT during CR1/PR1 remained a favorable factor for survival with an HR of 0.52 (p=0.02). Among 26 patients who died in cohort A at the study cut-off time, 14 patients (54%) died from relapsed PTCL post auto HCT.
Conclusion Our data showed that auto HCT during the first response was associated with longer survival in transplant-eligible patients with nodal PTCL who achieved CR or PR after first-line chemotherapy. This is despite the fact that 57% of the patients in cohort B received an auto HCT at a later time. Our results highlight a benefit to performing auto HCT during first remission instead of later in the disease course. Large studies are needed to validate our conclusion.
Disclosures
Saeed:Novartis: Consultancy; Epizyme: Consultancy; Morphosys: Honoraria. Shah:Servier: Other: grants and investigator-initiated trials; PeproMene Bio: Other: Steering committee; Autolus: Consultancy; Century Therapeutics: Consultancy; Adaptive: Consultancy; Pharmacyclics: Consultancy; Beigene: Consultancy; Acrotech: Consultancy; Jazz: Consultancy, Other: grants and investigator-initiated trials; Precision Biosciences: Consultancy; Kite/Gilead: Consultancy, Other: grants and investigator-initiated trials; BMS/Celgene/Juno: Consultancy; Novartis: Consultancy; Pfizer: Consultancy; Amgen: Consultancy. Lazaryan:Teladoc: Current equity holder in publicly-traded company; Humanigen: Consultancy; AvroBio: Consultancy; AmWel: Current equity holder in publicly-traded company; Sanofi: Consultancy. Pinilla Ibarz:SecuraBio: Research Funding; AstraZeneca: Consultancy; AbbVie: Consultancy; Pharmacyclics: Consultancy; Janssen Pharmaceuticals: Consultancy. Liu:Sanofi: Speakers Bureau. Locke:Takeda: Consultancy; CERo Therapeutics: Research Funding; ), National Cancer Institute: Research Funding; Leukemia and Lymphoma Society: Research Funding; Aptitude Health: Other: Education or editorial activity; ASH: Other: Education or editorial activity; BioPharm Communications: Other: Education or editorial activity; CAREducation: Other: Education or editorial activity; Clinical Care Options Oncology: Other: Education or editorial activity; Imedex: Other: Education or editorial activity; Society for Immunotherapy of Cancer: Other: Education or editorial activity; Sana: Consultancy; Daiichi Sankyo: Consultancy; BMS: Research Funding; A2: Consultancy; Celgene: Consultancy; Other: Patents & Royalties: patents, royalties, other intellectual property from several patents held by the institution in my name (unlicensed) in the field of cellular immunotherapy.; Wugen: Consultancy; Umoja: Consultancy; Novartis: Consultancy, Research Funding; Legend Biotech: Consultancy; Kite, a Gilead Company: Consultancy, Research Funding; Janssen: Consultancy; Iovance: Consultancy; GammaDelta Therapeutics: Consultancy; Emerging Therapy Solutions Gerson Lehrman Group: Consultancy; EcoR1: Consultancy; Cowen: Consultancy; Calibr: Consultancy; Cellular Biomedicine Group: Consultancy; Bristol Myers Squibb/Celgene: Consultancy; Bluebird Bio: Consultancy, Research Funding; Allogene: Consultancy, Research Funding; Amgen: Consultancy. Jain:MyeloidTx: Consultancy; BMS: Consultancy; Incyte: Research Funding; Novartis: Consultancy; Kite Pharma: Consultancy, Research Funding. Sokol:Kyowa-Kirin: Honoraria, Research Funding; Dren Bio: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.